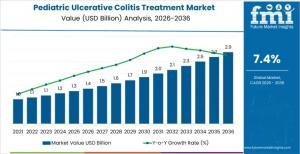
Pediatric Ulcerative Colitis Treatment Market to Reach USD 2.9 Billion by 2036 on Protocol-Led Adoption
Standardized care pathways and rising biologic adoption are driving the pediatric UC treatment market at a steady 7.4% CAGR.
NEWARK, DE, UNITED STATES, January 17, 2026 /EINPresswire.com/ -- Pediatric Ulcerative Colitis Treatment Market Shows Stable, Clinically Anchored Growth
The Pediatric Ulcerative Colitis Treatment Market is forecast to expand from USD 1.4 billion in 2026 to USD 2.9 billion by 2036, registering a CAGR of 7.4%. Unlike consumer-driven healthcare segments, demand growth is tied to standardized pediatric gastroenterology protocols, long-term disease management needs, and increasing access to advanced therapies across hospital networks and specialty clinics.
Clinical adoption patterns, rather than brand visibility, shape market momentum. Once a therapy is incorporated into pediatric treatment guidelines, switching requires additional trials, regulatory reassessment, and payer approval, reinforcing long usage cycles and predictable demand. As a result, market expansion reflects cumulative uptake across healthcare systems instead of rapid competitive displacement.
Subscribe for Year-Round Insights → Stay ahead with quarterly and annual data updates:
https://www.futuremarketinsights.com/reports/sample/rep-gb-31415
Clinical Decision-Making Defines Market Demand
Treatment selection in pediatric ulcerative colitis is governed by disease severity, patient age, safety profile, and route of administration. Pediatric gastroenterologists prioritize therapies that support sustained remission while minimizing growth-related side effects and long-term complications.
Key clinical drivers include:
- Guideline compliance and protocol alignment
- Demonstrated efficacy in pediatric populations
- Safety and tolerability over multi-year treatment cycles
- Administration convenience for caregivers and patients
Hospitals and specialty clinics integrate these therapies into structured care pathways, linking drug consumption directly to enrolled patient volumes rather than episodic outpatient visits.
Commercial Dynamics Follow Care Pathways, Not Promotion
Commercial success in this market mirrors clinical adoption rather than aggressive marketing strategies. Manufacturers focus on formulation consistency, delivery device reliability, and storage stability to meet hospital requirements. Release protocols emphasize sterility testing, potency verification, and expiry confirmation before staged distribution to regional hospitals and pediatric centers.
Key operational priorities for suppliers include:
- Reliable cold-chain and inventory management
- Predictable reorder cycles aligned with treatment schedules
- Technical support for dosing and administration
- Patient and caregiver education to support adherence
Profitability is therefore driven by continuity of care and low wastage, rather than discount-led volume growth.
Biologic Therapies Lead Product Adoption
Biologic therapies account for approximately 46% of market demand, reflecting their role in managing moderate to severe pediatric ulcerative colitis. Their targeted mechanisms offer improved disease control, making them central to modern pediatric treatment protocols.
Product-level adoption trends include:
- Biologics: High efficacy, infusion or injectable administration, strong protocol integration
- Small-molecule drugs: Oral options improving adherence and outpatient management
- Steroids and 5-ASAs: Ongoing use for mild disease and flare control
Once product classes are embedded in clinical workflows, switching remains limited due to stability concerns and reimbursement structures.
Application Mix Creates Predictable Demand Streams
Moderate ulcerative colitis cases represent roughly 54% of total demand, as consistent therapy is essential to prevent progression and hospitalization. Severe cases, while smaller in volume, generate higher value due to combination therapy use, monitoring intensity, and specialized infrastructure requirements.
This application mix results in:
- Stable baseline demand from moderate cases
- High-value opportunities in severe disease management
- Long-term supplier engagement through adherence and monitoring support
Hospitals align procurement strategies with disease severity distribution rather than short-term purchasing trends.
Asia Pacific Emerges as the Fastest-Growing Region
Geographically, Asia Pacific leads growth due to expanding pediatric healthcare infrastructure and earlier diagnosis of inflammatory bowel disease.
Country-level growth highlights include:
- India: 11.3% CAGR, driven by private hospital expansion and biologic access
- China: 11.1% CAGR, supported by national hospital networks and chronic care programs
- Brazil: 10.2% CAGR, reflecting growth in private pediatric networks
- United States: 9.6% CAGR, shaped by protocol updates and program expansion
- Germany: 8.1% CAGR, reflecting steady adoption in a mature, guideline-driven system
Competitive Landscape Centers on Clinical Evidence
Leading players such as AbbVie, Johnson & Johnson, Pfizer, Takeda, and Bristol Myers Squibb compete primarily on pediatric trial data, safety outcomes, and protocol inclusion. Competitive advantage is established during formulary evaluation rather than post-launch promotion.
Hospitals assess total value based on:
- Reduction in flares and hospitalizations
- Ease of integration into existing care pathways
- Support services for clinicians and caregivers
Outlook: Growth Anchored in Long-Term Care Models
The pediatric ulcerative colitis treatment market will continue expanding through 2036 as standardized care models, earlier diagnosis, and biologic adoption advance globally. Suppliers that align with clinical workflows, provide consistent supply, and support adherence are positioned for sustained growth.
Get data that aligns with your strategic priorities — ask for report customization today:
https://www.futuremarketinsights.com/customization-available/rep-gb-31415
Related Reports
Radiation-Free Fetal Heart Rate Monitor Market – https://www.futuremarketinsights.com/reports/radiation-free-fetal-heart-rate-monitor-market
Small Molecule CDMO Market – https://www.futuremarketinsights.com/reports/small-molecule-cdmo-market
Conversational AI in Healthcare Market – https://www.futuremarketinsights.com/reports/conversational-ai-in-healthcare-market
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us - sales@futuremarketinsights.com
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware - 19713, USA
T: +1-347-918-3531
Why FMI: https://www.futuremarketinsights.com/why-fmi
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.